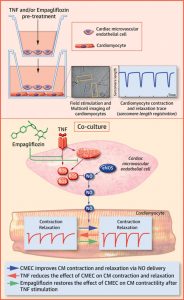
Cardiac Microvascular Endothelial Enhancement of Cardiomyocyte Function Is Impaired by Inflammation and Restored by Empagliflozin
Highlights
-
• Cardiac microvascular endothelial cells (CMECs) exert a direct positive effect on cardiomyocyte contraction and relaxation, which is mainly mediated by endothelial-derived nitric oxide (NO).
-
• Pro-inflammatory stimulation of CMECs by pre-incubation with tumor necrosis factor-α (TNF-α) or interleukin-1β abrogates the positive regulatory function of these cells on cardiomyocyte contractile property.
-
• Mechanistically, pro-inflammatory activation of CMECs leads to mitochondrial and cytoplasmic reactive oxygen species (ROS) accumulation that results in the scavenging of NO.
-
• Empagliflozin directly restores the beneficial effect of CMECs on cardiomyocyte contraction and relaxation by reducing TNF-α-induced mitochondrial and cytoplasmic ROS accumulation, which leads to reinstatement of CMEC-derived NO delivery.
Summary
The positive findings of the EMPA-REG OUTCOME trial (The randomized, placebo-controlled cardiovascular outcome trial of empagliflozin) on heart failure (HF) outcome in patients with type 2 diabetes mellitus suggest a direct effect of empagliflozin on the heart. These patients frequently have HF with preserved ejection fraction (HFpEF), in which a metabolic risk-related pro-inflammatory state induces cardiac microvascular endothelial cell (CMEC) dysfunction with subsequent cardiomyocyte (CM) contractility impairment. This study showed that CMECs confer a direct positive effect on contraction and relaxation of CMs, an effect that requires nitric oxide, is diminished after CMEC stimulation with tumor necrosis factor-α, and is restored by empagliflozin. Our findings on the effect of empagliflozin on CMEC-mediated preservation of CM function suggests that empagliflozin can be used to treat the cardiac mechanical implications of microvascular dysfunction in HFpEF.
http://basictranslational.onlinejacc.org/content/early/2019/08/27/j.jacbts.2019.04.003